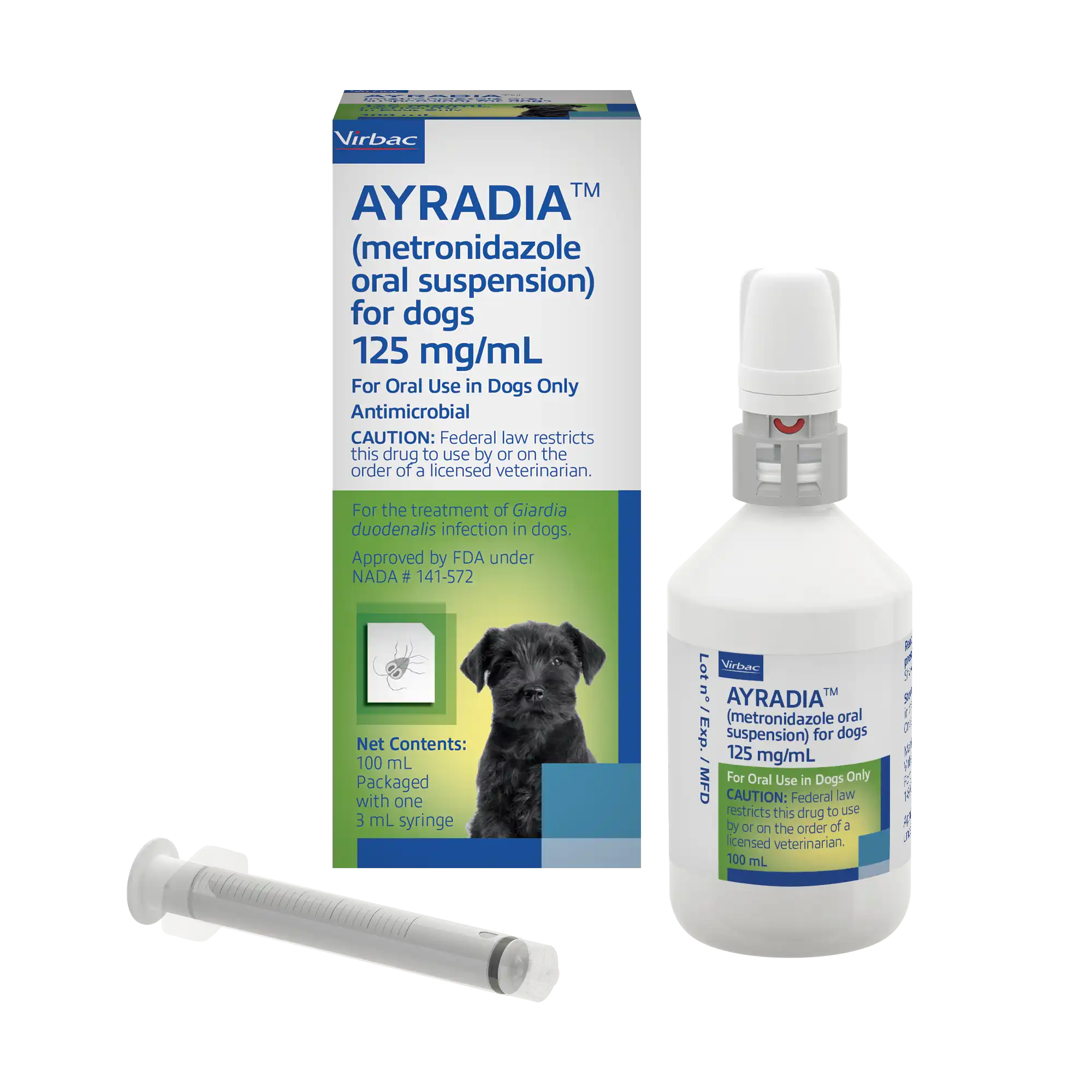
AYRADIA™ (metronidazole oral suspension) for dogs
AYRADIA™ oral suspension The first FDA-approved flavored liquid metronidazole for veterinary use.
Proven effectiveness for the treatment of giardiasis in dogs. The flavored liquid formulation of AYRADIA oral solution makes dosing easy and accurate for dogs and puppies of all sizes.
.webp)
AYRADIA oral suspension is indicated for the treatment of Giardia duodenalis infection in dogs.
GIARDIA IS A PROTOZOAL PARASITE THAT:
- May be present in nonclinical healthy dogs1
- Presents clinically as gastrointestinal infections commonly causing diarrhea that may be severe in some cases1
- Is a contagious disease and may be contracted by animals exposed to others shedding the parasite in their feces1
- May be zoonotic,1 particularly in young and immunosuppressed people
The flavored liquid formulation of AYRADIA oral suspension:
AYRADIA oral suspension has demonstrated bioequivalence compared with metronidazole tablets2
Ref: Pellet T, Toutin C, Eynard I, Fournel S, Gruet P, (2018) Bioequivalence study between an oral suspension and a tablet formulation of metronidazole following a single oral administration in dogs, EAVPT Congress
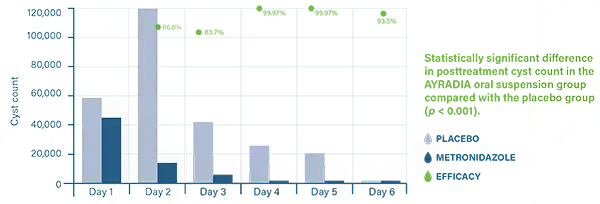
AYRADIA decreases infective cyst excretion and intestinal trophozoites in dogs infected with Giardia duodenalis.3,4
A DELIVERY SYSTEM DESIGNED FOR PRACTICALITY, SAFETY AND SIMPLICITY
Features of the AYRADIA delivery system:
![Araydia-smartcap_icon.webp]()
-
SMARTCAP LID IS CHILDPROOF AND SENIOR-FRIENDLY
![Araydia-shelf-life_icon.webp]()
-
THREE-YEAR SHELF LIFE Use within 6 months of opening
![Araydia-refridge_icon.webp]()
-
REFRIGERATION NOT REQUIRED
![Araydia-bottles_icon.webp]()
-
AVAILABLE IN 30 ML AND 100 ML BOTTLES
Instructions for Using the Dispensing System and Preparing a Dose of AYRADIA™ (metronidazole oral suspension)
Risk of No FDA Review and Monitoring
Because unapproved animal drugs don’t go through FDA's pre-market review, veterinarians, pet owners, and animal producers have no way to know if these drugs are safe and effective or if their manufacturing processes are adequate to maintain quality and consistency from batch to batch. Also, because drug companies aren’t required to report adverse drug experiences and product defects for unapproved animal drugs to FDA, problems with these drugs may be slow to be identified.5
![23_icon_dark.png]() Shop Vetcove on Virbac
Shop Vetcove on Virbac
You can shop all Virbac products on Vetcove and connect your distributor accounts to purchase and access rewards.
Pet Owner Information:
We have a separate site specifically tailored to pet owners with all the information you need to ensure your pets are taken care of to the highest degree! Click here to view this product's page on our pet owner website!
Important Safety Information:
AYRADIA™ (metronidazole oral suspension): Not for use in humans. Avoid contact with skin and wash hands after use. In dogs, neurologic effects have been associated with AYRADIA oral suspension use at high doses. Use with caution in dogs with hepatic dysfunction. The safe use of this drug in dogs intended for breeding purposes and in pregnant or lactating bitches has not been evaluated. For the full prescribing information, call Virbac at 1-800-338-3659 or view the Product Insert.
References:
- FDA approves first treatment for Giardia duodenalis in any animal species. FDA. UpdatedOctober25,2023.Accessed November 11, 2023.https://www.fda.gov/animal-veterinary/cvm-updates/fda-approves-first-treatment-giardia-duodenalis-anyanimal-species.
- Pellet T, Toutin C, Eynard I, Fournel S, Gruet P. Bioequivalence study between an oral suspension and a tablet formulation of metronidazole following a single oral administration in dogs. Poster presented at: EAVPT Congress; June 24-27, 2018; Wrocław, Poland. doi:10.1111/jvp.12653
- Data on file. Virbac Corporation.
- Bowman DD, Nicolas C, Navarro C, et al. A pilot study to determine the efficacy of a metronidazole suspension on the reduction of cyst and trophozoite counts in dogs infested with Giardia. Abstract presented at: 27th Conference of the World Association for the Advancements of Veterinary Parasitology; July 7-11, 2019; Madison, WI. Abstract PS01.34:206.
- Medicine, C. for V. (n.d.). FDA’s concerns about unapproved animal drugs. U.S. Food andDrugAdministration.https://www.fda.gov/animal-veterinary/unapproved-animal-drugs/fdas-concerns-about-unapproved-animal-drugs