EUTHASOL® (pentobarbital sodium and phenytoin sodium) Euthanasia Solution Important Safety Information
WARNING: For canine euthanasia only. Must not be used for therapeutic purposes. Do not use in animals intended for food.
CAUTION: Caution should be exercised to avoid contact of the drug with open wounds or accidental self‐inflicted injections.
Keep out of reach of children. If eye contact, flush with water and seek medical advice/attention. Euthanasia may be delayed in
dogs with severe cardiac or circulatory deficiencies.
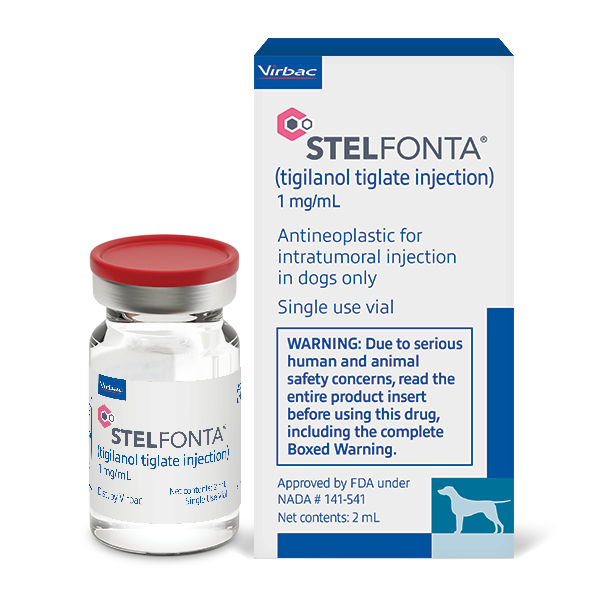
STELFONTA® (tigilanol tiglate injection) IMPORTANT SAFETY INFORMATION
Accidental self-injection of STELFONTA® may cause severe wound formation. To decrease the risk of accidental self-injection, sedation of the dog may be necessary. In dogs, do not inject STELFONTA into subcutaneous mast cell tumors located above the elbow or hock. Formation of wounds, possibly extensive, is an intended and likely response to treatment with STELFONTA along with associated swelling, bruising and pain; these wounds are expected to heal. Appropriate pre- and post-treatment medications must be given, including a corticosteroid plus blocking agents for both H1 and H2 receptors, in order to decrease the potential for severe systemic adverse reactions, including death, from mast cell degranulation. For full prescribing information, contact VIRBAC at 1-800-338-3659 or view the Product Insert.
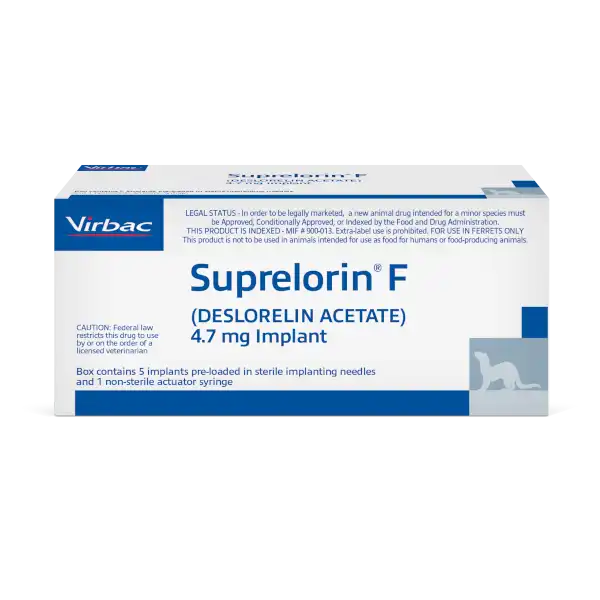
SUPRELORIN® F (deslorelin acetate) Implant (4.7 mg) Important Safety Information:
For use in ferrets only. Do not use in animals intended for breeding. The safe use of this product has not been evaluated in pregnant or lactating ferrets. Do not use this product in ferrets with known hypersensitivity to deslorelin acetate or other synthetic hormones. DO NOT HANDLE THIS PRODUCT IF YOU ARE PREGNANT OR NURSING OR SUSPECT YOU MAY BE PREGNANT. Accidental administration in humans may lead to disruption of the menstrual cycle. For complete product information phone Virbac at 1-800-338-3659 or download here.
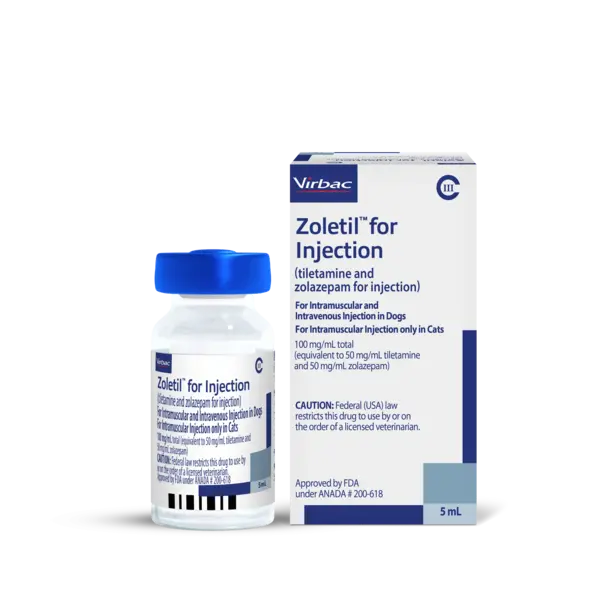
ZoletilTM for Injection (tiletamine and zolazepam for injection) Important Safety Information:
ZoletilTM for Injection (tiletamine and zolazepam for injection) should not be used : 1) in dogs and cats with severe cardiac or pulmonary dysfunction, or pancreatic disease; 2) at any stage of pregnancy or for Cesarean section; 3) in cats suffering from renal insufficiency; 4) with phenothiazine-derivative drugs as the combination produces respiratory and myocardial depression, hypotension, and hypothermia. Pulmonary edema has been reported in cats. Respiratory depression may occur following administration of high doses. Copious salivation that may occur during anesthesia can be controlled by concurrent administration of atropine sulfate. Reduce dosage in geriatric dogs and cats. Patients should be continuously monitored. For the full prescribing information download here or call 1-800-338-3659 or download here.