Welcome to STELFONTA®
(tigilanol tiglate injection)
STELFONTA®: An innovative and effective treatment for Mast Cell Tumors in dogs
Complete
responseWound heals via
second intention*Rapid return of
quality of life
from disease
* Minimal intervention. Bandaging and antibiotics usually not required.
STELFONTA® removes 75% of canine Mast Cell Tumors with a single treatment*
87% of dogs had a complete response* after either one or two treatments combined**
RESPONSE
* Complete response was defined as complete removal of the tumor.Eisenhauer EA, et al. European Journal of Cancer 2009;45(2):228–47.
** Results from pivotal field efficacy study: 68/78 dogs achieved a complete response at 28 days after one or two treatments of STELFONTA®.
89% of dogs disease-free* at 12 monthsJones, PD, Campbell, JE, Brown, G, Johannes, CM, Reddell, P. Recurrence‐free interval 12 months after local treatment of mast cell tumors in dogs using intratumoral injection of tigilanol tiglate. J Vet Intern Med. 2020; 1– 5. https://doi.org/10.1111/jvim.16018
FREE
* At the site of STELFONTA treatment.
Study design: A multi-center, randomized, controlled, investigator- and owner-masked clinical study in 123 client-owned dogs with MCT measuring ≤10cm3. Effectiveness was evaluated using response evaluation criteria in solid tumors (RECIST), where complete response was defined as complete removal of the tumor.Eisenhauer EA, et al. European Journal of Cancer 2009;45(2):228–47. Dogs in the STELFONTA® group were treated once at the start of the study, in addition to receiving concurrent medications. Patients in the STELFONTA® treated or the control group that did not achieve a complete response at Day 28 were eligible to receive a second treatment or a first treatment if the patient was in the original control group. All patients in both the STELFONTA® treated and the control group received concurrent medications. Patients that achieved a complete response at Day 28 in either phase were followed for 12 weeks after the final treatment. Where possible, patients were assessed at 12 months for tumor recurrence.

STELFONTA® Indication
FDA-approved local treatment for non-metastatic Mast Cell Tumors
Indicated for the treatment of:
STELFONTA® (tigilanol tiglate injection) is an intratumoral injection indicated for use in dogs for the local treatment of MCTs that are:
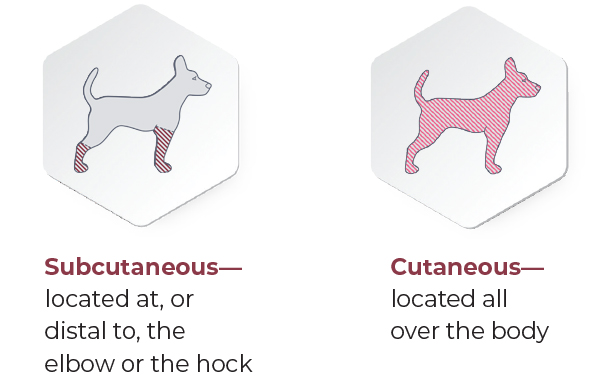
- Non-metastatic subcutaneous mast cell tumors located at or distal to the elbow or the hock in dogs
- Non-metastatic cutaneous mast cell tumors located all over the body
- Tumors must be less than or equal to 10 cm3 in volume, and must be accessible to intratumoral injection
- Do not exceed 5 mL per dog, regardless of tumor volume or body weight
- The minimum dose of STELFONTA is 0.1 mL, regardless of tumor
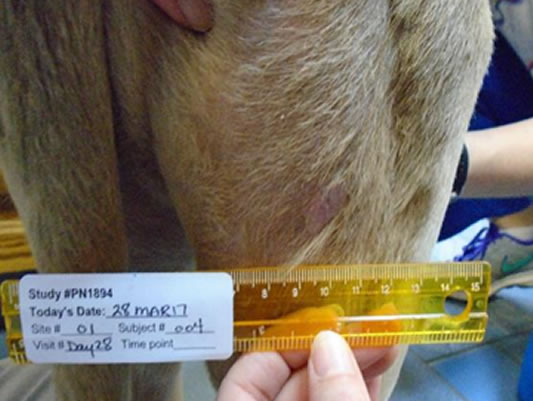
See STELFONTA® start to work within hours,Melo SR, et al. Veterinary Cancer Society, Houston, Texas, USA. with tumors typically destroyed by Day 7
-
![]()
Day 1 Acute inflammatory response with swelling and erythema noted on the tumor margins and surrounding tissues.Melo SR, et al. Veterinary Cancer Society, Houston, Texas, USA.
-
![]()
Day 7 Necrotic destruction is seen – in dogs this means blackening, shrinkage and leakage of thick discharge
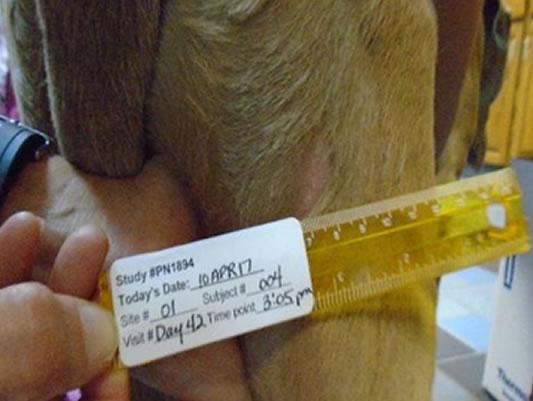
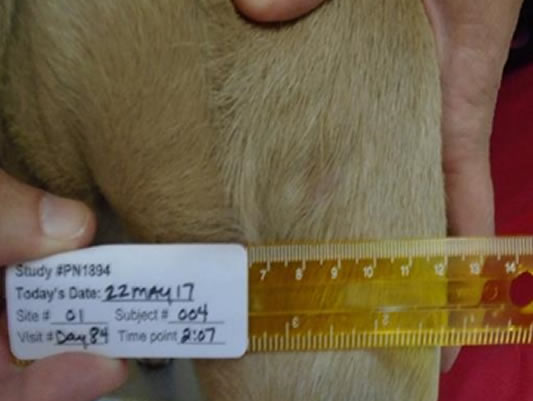
Second intention healingReddell, P, De Ridder, TR, Morton, JM, et al. Wound formation, wound size, and progression of wound healing after intratumoral treatment of mast cell tumors in dogs with tigilanol tiglate. J Vet Intern Med. 2021; 35: 430– 441. https://doi.org/10.1111/jvim.1600 with more than half of tumor sites fully healed by Day 28
-
![]()
Day 28 57% wounds fully healed
-
![]()
Day 42 78% wounds fully healed
-
![]()
Day 84 96% wounds fully healed
Contact your veterinarian if you notice any of the following changes in your dog:
-
Excessive pain or lameness (limping)
-
Tiredness or refusal to eat for more than 1 day
-
Repeated vomiting or diarrhea
-
Trouble breathing
-
Changes to the treated tumor site, including increased or excessive swelling and bruising, extensive wound formation, or increased irritation
-
Any other symptoms that your dog may show that concern you.
Dog owners report STELFONTA® treatment doesn’t negatively impact quality of lifeMiller J, et al JVIM 2020; article accepted in press.
“… By following the treatment process to the letter, Daisy coped exceptionally well, and it’s now 18 months later with no recurrence, and her quality of life is excellent. Daisy is now 10 and enjoying her later years with typical Jack Russell zest, energy and appetite!”
Brigitte and Brian Wright
Owners of Daisy

How observing natural defenses in nature resulted in discovering STELFONTA®
![]() DISCOVERY
DISCOVERY
STELFONTA® was discovered by Australian company QBiotics, who found a new biologically active chemical (tigilanol tiglate) in the seed of the Australian native blushwood plant (Fontainea picrosperma).
What are some possible side effects of STELFONTA® (tigilanol tiglate injection)?
- STELFONTA may cause side effects, even at the prescribed dose. These side effects include, but are not limited to:
- During the first days after treatment, you may see bruising or swelling around the treated tumor site. The swelling may cause your dog some discomfort and pain for several days after treatment. Your dog may seem tired during this time and may eat less.
- In some cases, a severe, larger than normal wound may develop, delaying wound healing. Your veterinarian will assess if your dog requires additional treatments during this time (e.g. bandages, Elizabethan collar).
- Other side effects may occur. For more information about side effects ask your veterinarian.
Common adverse events
- Most common adverse events such as wound formation, injection site pain/bruising/erythema/edema, lameness in a treated limb are related to STELFONTA®’s mode of action at the tumor site
- Formation of wounds is a secondary intention to treatment with STELFONTA®
Common adverse events seen following STELFONTA® treatment
- Signs due to mode of action include:
-
- Wound formation (possibly extensive) following tumor necrosis and slough
- Localized swelling, erythema, bruising at the tumor site
- Pain at the treated site
- Lameness in a treated limb
- Regional lymph node enlargement
- Signs that might be caused by concurrent medication include:
-
- Polyuria
- Polydispsia
- Tachypnea
- Increased appetite
- Elevated alkaline phosphatase
- Signs of degranulation of the mast cell tumor:
-
- Vomiting
- Diarrhea
- Lethargy
- Anorexia/hyporexia
- Altered breathing/tachypnea
- Bruising and edema at or away from the treated site
- Tachycardia
- Hypotension
For information on contraindications and warnings,
please refer to the Product Insert
|
WARNING: SEVERE WOUND FORMATION IN HUMANS; EXTENSIVE WOUND FORMATION, MAST CELL DEGRANULATION, AND DEATH IN DOGS DUE TO MAST CELL DEGRANULATION Human Safety
Dog Safety
|
IMPORTANT SAFETY INFORMATION
FOR VETERINARIANS
Accidental self-injection of STELFONTA® may cause severe wound formation. To decrease the risk of accidental self-injection, sedation of the dog may be necessary. In dogs, do not inject STELFONTA into subcutaneous mast cell tumors located above the elbow or hock. Formation of wounds, possibly extensive, is an intended and likely response to treatment with STELFONTA along with associated swelling, bruising and pain; these wounds are expected to heal. Appropriate pre- and post-treatment medications must be given, including a corticosteroid plus blocking agents for both H1 and H2 receptors, in order to decrease the potential for severe systemic adverse reactions, including death, from mast cell degranulation. For full prescribing information, contact VIRBAC at 1-800-338-3659 or view the Product Insert.
FOR PET OWNERS
Wear disposable gloves when cleaning the treated tumor site to avoid contact with any residual drug. Thoroughly wash your skin that comes in contact with the treated tumor site, wound, or wound discharge. Ensure your dog receives their prescribed medications to decrease the potential for severe, life-threatening adverse reactions. Monitor your dog during the healing process and contact your veterinarian if you notice excessive pain, lameness, tiredness, refusal to eat for more than one day, repeated vomiting or diarrhea, trouble breathing, changes to the treated tumor site (including increased or excessive swelling and bruising, extensive wound formation, increased irritation) or any other symptoms that concern you. For full prescribing information, contact Virbac at 1-800-338-3659 or view the Product Insert.
Pet Owner Information:
We have a separate site specifically tailored to pet owners with all the information you need to ensure your pets are taken care of to the highest degree! Click here to view this product's page on our pet owner website!