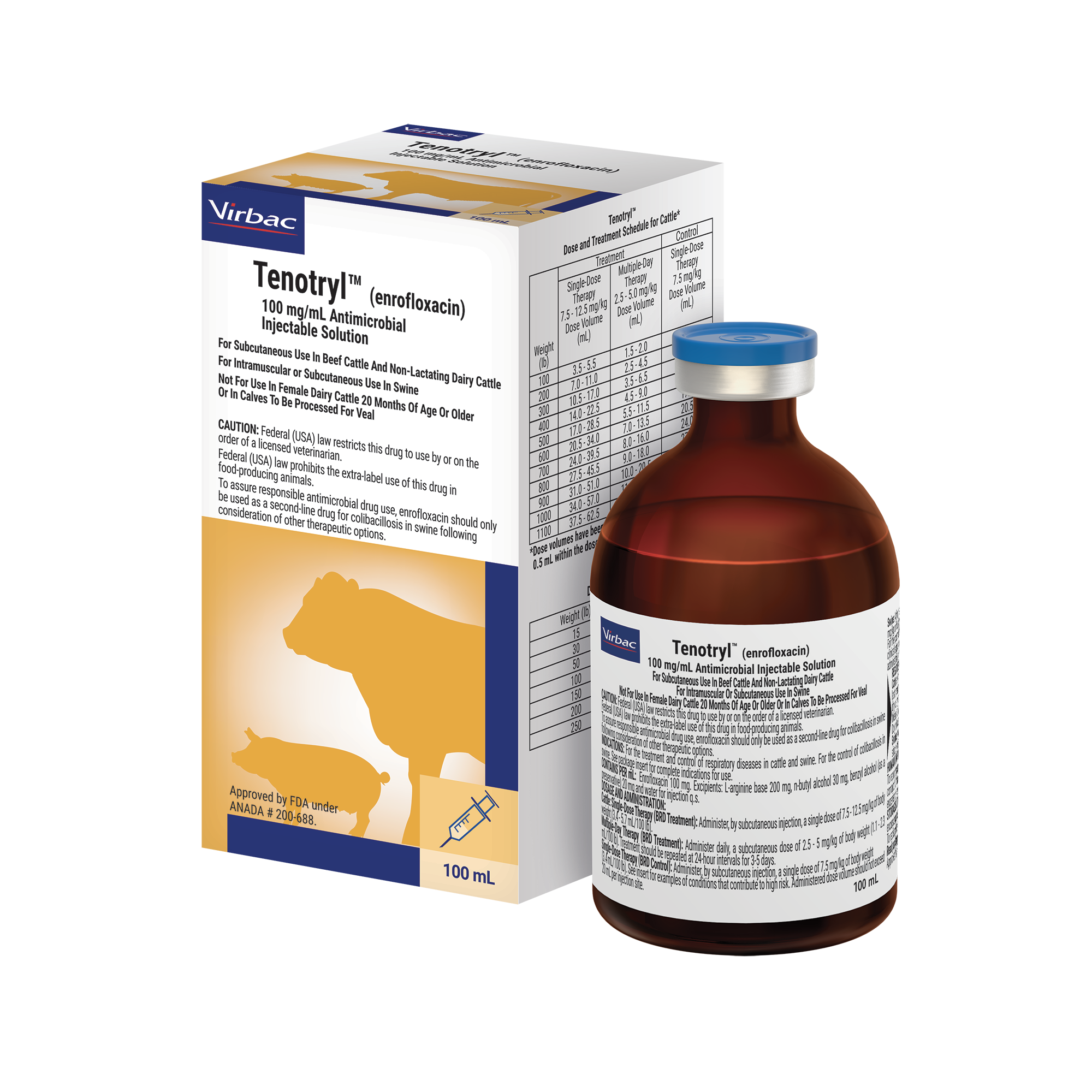
Tenotryl™ (enrofloxacin) injectable solution (Bovine)
For the treatment of bovine respiratory disease (BRD) associated with Pasteurella multocida, Mannheimia haemolytica, Histophilus somni and Mycoplasma bovis.
See more details for Swine Usage of TENOTRYL
See more details for Cattle Usage of TENOTRYL
In cattle only, Tenotryl™ works quickly at the infection site with enrofloxacin reaching peak plasma concentration in less than two hours.1 It remains active because ciprofloxacin has a maximum residence time of 13.7 hours.1

Designed to be fast and reliable

A single injection of enrofloxacin has a relatively brief period of effect. After a single subcutaneous injection of 12.5 mg/kg, the combined, unbound concentrations of enrofloxacin and ciprofloxacin are below the minimal inhibitory count (MIC) for 90% of Mannheimia haemolytica isolates in plasma and tissue fluids by 48 hours.2 Do not exceed a 20 mL dose per injection site. Subcutaneous injection in cattle can cause a transient local tissue reaction and may result in trim loss of edible tissue at slaughter.
Brief Summary of Cattle information
Brief Summary of Swine Information
Tenotryl™ Dose and Treatment Schedule for Cattle*

Important Safety Information
SWINE IMPORTANT SAFETY INFORMATION
Tenotryl™ (enrofloxacin) 100 mg/ml Antimicrobial Injectable Solution: Not for use in humans. For intramuscular or subcutaneous use in swine. Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian and prohibits the extra-label use of this drug in food producing animals. To assure responsible antimicrobial drug use, enrofloxacin should only be used as a second-line drug for colibacillosis in swine following consideration of other therapeutic options. Animals intended for human consumption must not be slaughtered within 5 days of receiving a single injection dose. The effects of enrofloxacin on swine reproductive performance, pregnancy and lactation have not been adequately determined. The long-term effects on articular joint cartilage have not been determined in pigs above market weight. Subcutaneous or intramuscular injection in swine can cause a transient local tissue reaction and may result in trim loss of edible tissue at slaughter. Quinolone-class drugs should be used with caution in animals with known or suspected Central Nervous System (CNS) disorders.
CATTLE IMPORTANT SAFETY INFORMATION
Tenotryl™ (enrofloxacin) 100 mg/ml Antimicrobial Injectable Solution: Not for use in humans. For subcutaneous use in beef cattle and non-lactating dairy cattle. Not for use in female dairy cattle 20 months of age or older or in calves to be processed for veal. Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian and prohibits the extra-label use of this drug in food producing animals. Animals intended for human consumption must not be slaughtered within 28 days from the last treatment. This product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in the calves born to these cows. A withdrawal period has not been established for this product in pre-ruminating calves. The effects of enrofloxicin on cattle reproductive performance, pregnancy and lactation have not been adequately determined. Do not exceed a 20 mL dose per injection site. Subcutaneous injection in cattle can cause a transient local tissue reaction and may result in trim loss of edible tissue at slaughter. Quinolone-class drugs should be used with caution in animals with known or suspected Central Nervous System (CNS) disorders.