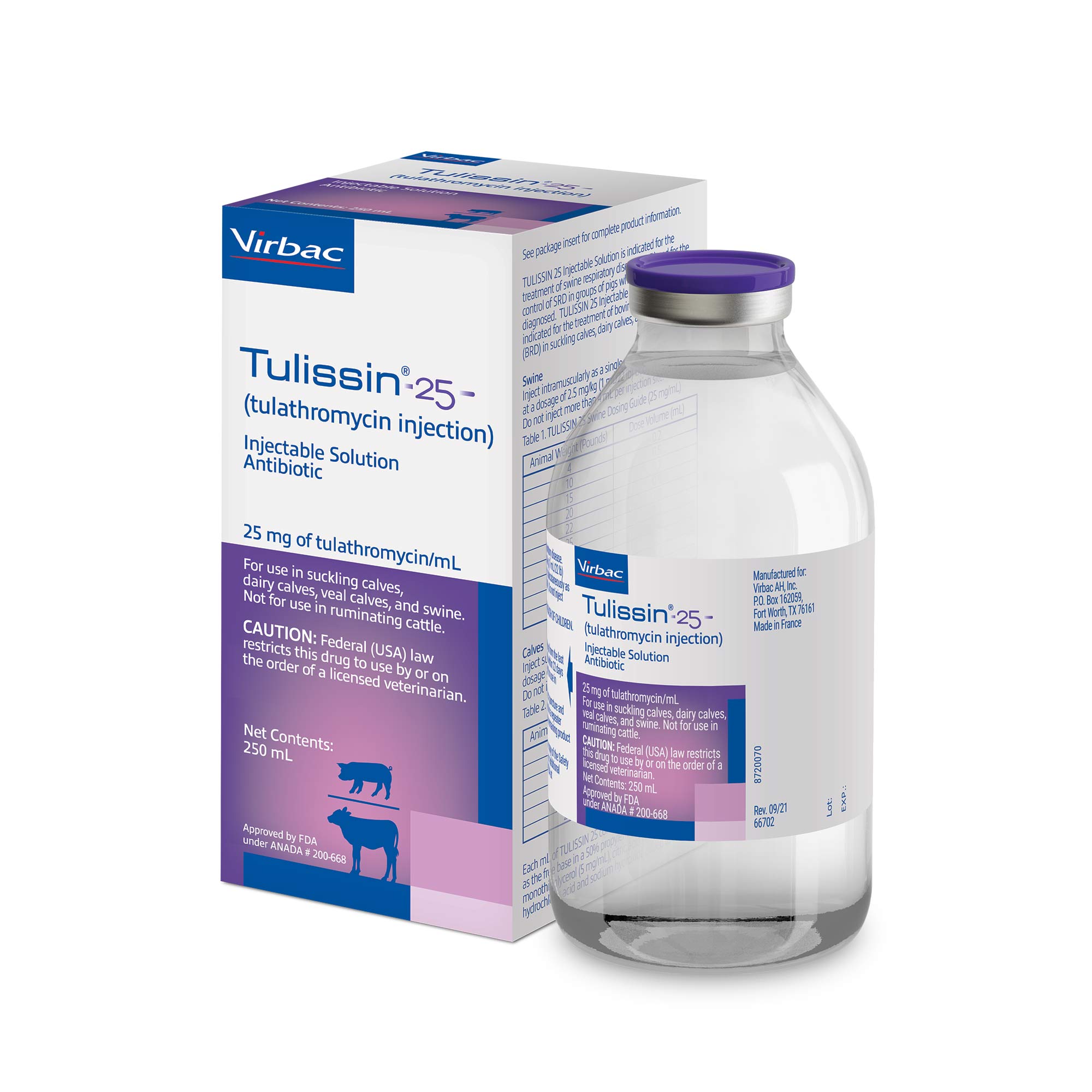
Tulissin® 100 (tulathromycin injection) injectable solution (Cattle)
Protect Your Investment
See additional content for Tulissin® for Cattle
See additional content for TULISSIN for Swine
Tulissin® 100 Injectable Solution isn’t just for BRD
-
Reach for it in the face of Infectious Bovine Keratoconjunctivitis (IBK), more commonly known as pinkeye, when associated with Moraxella Bovis. This drug is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows.
-
Tulissin® 100 Injectable Solution is also indicated for bovine foot rot (interdigital necrobacillosis) associated with either Fusobacterium necrophorum and Porphyromonas levii. Note that Tulissin® 100 Injectable Solution is contraindicated in animals previously found to be hypersensitive to the drug.
![infography-photo-dog.jpg]() Packaged to protect your investment
Packaged to protect your investment
- After 3 years of development, our patented2 protective shell offers excellent shock absorption properties.
- Studies3 have shown 92% of the bottles resisted breakage when dropped 3 times from a height of up to 4 feet!
- Virbac’s protective shell can be found on Tulissin® 100 Injectable Solution 250 mL and 500 mL bottles.
Brief Summary of Cattle Information
Brief Summary of Swine Information
More information on sizes

Important Safety Information
IMPORTANT SAFETY INFORMATION FOR CATTLE
TULISSIN® 100 (tulathromycin injection): Not for use in humans. Ensure a pre-slaughter withdrawal time of eighteen (18) days in cattle. Do not use in dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. The effects of tulathromycin on bovine reproductive performance, pregnancy and lactation have not been determined. Do not use in animals known to be hypersensitive to the product.
IMPORTANT SAFETY INFORMATION FOR SWINE
TULISSIN® 100 (tulathromycin injection): Ensure a pre-slaughter withdrawal time of five (5) days in swine. The effects of tulathromycin on swine reproductive performance, pregnancy and lactation have not been determined. Do not use in animals known to be hypersensitive to the product.


 Packaged to protect your investment
Packaged to protect your investment