Success Stories
Discover the STELFONTA® (tigilanol tiglate injection) treatment journey for pet owners
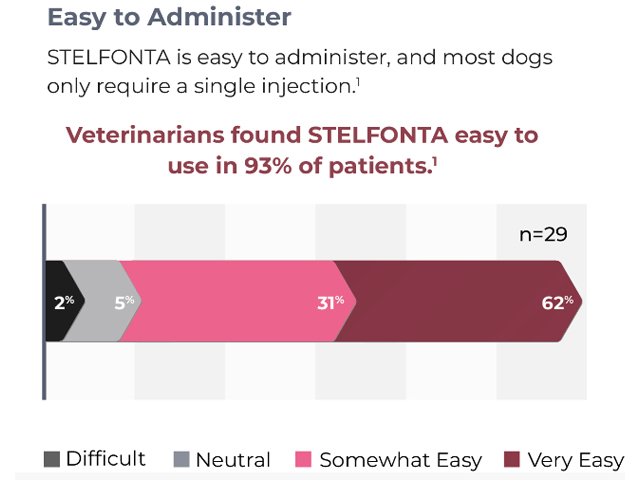
![Stelfonta_Chart_B2B.jpg]()
Discover the STELFONTA® (tigilanol tiglate injection) treatment journey for pet owners
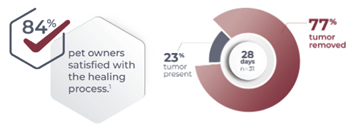
-
![]()
It worked out wonderfully -
![]()
Back to her normal self with STELFONTAⓇ (tigilanol tiglate injection) -
![]()
She got her wiggle back with STELFONTAⓇ (tigilanol tiglate injection) -
![]()
The healing process was quite good. I am very pleased with STELFONTAⓇ (tigilanol tiglate injection) -
![]()
We have been happy with STELFONTAⓇ (tigilanol tiglate injection) -
![]()
Turnip is doing AMAZING, thanks to STELFONTAⓇ (tigilanol tiglate injection), living her BEST life -
![]()
It's a great option for our patients with mast cell tumors -
![]()
Almost 20 patients treated with STELFONTAⓇ (tigilanol tiglate injection) -
![]()
Amazing to watch -
![]()
Back to being a typical Staffy bullet -
![]()
Extended her life -
![]()
Even better than before -
![]()
Saved Daisy's leg -
![]()
Thank you for recommending STELFONTAⓇ (tigilanol tiglate injection) -
![]()
Glad my veterinarian recommended an injectable -
![]()
Talk about the process with your veterinarian -
![]()
Bounced back to normal -
![]()
Treated what surgery could not -
![]()
11-year-old, well after treatment
It worked out wonderfully
Watch Mary and Dale's story
When 12-year-old Dale was diagnosed with a mast cell tumor, Mrs. Forsythe’s veterinarian recommended STELFONTAⓇ (tigilanol tiglate injection). For this pet owner, seeing was believing.
“I was fascinated to see the healing process. Within hours of the injection, it started to look different. And then it was the second or third day her leg swelled up. It swelled up and she was a little lame, then the leg went down and the tumor started to slough off. Within a couple days, it started getting yucky, but it wasn’t messy. Within a couple of weeks, it was healed over. It took a couple months to completely close up, and now the hair is growing in. It never got infected, never got dirty, never looked bad or anything. It kind of took care of itself.”
Dale wasn’t fazed by the healing process.
“We didn’t need to cone Dale. She never yelped or complained. It never bothered her.”
Treatment with STELFONTA has been associated with cellulitis and severe tissue sloughing extending away from the treated site, resulting in extensive wounds that require additional treatment and prolonged recovery time.
Watch Mary and Dale's story

Back to her normal self with STELFONTAⓇ (tigilanol tiglate injection)
Honey and her owner Mrs. Lori Weber live in St. Augustine, FL. Honey was diagnosed with a mast cell tumor while another dog in the household was undergoing surgery. Their veterinarian recommended STELFONTA as the best option to treat Honey’s mast cell tumor.
“Knowing that we had another dog that was going to be healing, [Dr. Bendick] thought that STELFONTA was a good option. It is an injection. After she was given a mild sedative, Honey was back and moving in about half an hour.”
"The wound was gross I have to say. It did not seem to bother Honey. We just kind of dabbed it clean here and there. She did her own things like she always does. It didn’t affect her mobility, her eating, nothing.”
Mrs. Weber was satisfied with the overall results of STELFONTA and would recommend it to other pet owners as an alternative to surgery.
“Surgeries are just so rough and Honey’s an older dog. It really was an amazing solution for our situation. I would recommend it.”
Appropriate pre- and post-treatment medications must be given in order to decrease the potential for severe systemic adverse reactions, including death, from mast cell degranulation.

She got her wiggle back with STELFONTAⓇ (tigilanol tiglate injection)
Dixie was diagnosed with a mast cell tumor when she was 7 years of age. Due to the location, her veterinarian did not think they could operate and achieve the necessary margins. The alternative was amputation and radiation.
Dixie's owner, Ally Heck said, “My husband did some research and learned about STELFONTA. We ended up going to a different veterinarian who was willing to try it. Dixie went through the procedure beautifully without sedation."
"When we took her home it had already swollen, she was limping around and not herself for the first 2-3 days, it took about a week for her to be normal. We watched her leg to make sure the swelling didn’t increase further, and the more she walked on it, the more it went down. We wore rubber gloves when dealing with it; after a week it was gross but healing well."
"Another one of our dogs got a lump on the hip which was not a mast cell tumor and had to have surgery to have that removed. Definitely a different experience managing that as he had to go under anesthesia, go back for stitches, etc. I would choose STELFONTA treatment again for a mast cell tumor.”
STELFONTA should not be injected into subcutaneous mast cell tumors located above the elbow or hock (e.g., on the body, head or neck), as this may result in accumulation of necrotic debris in the subcutaneous space, increasing the risk of systemic adverse reactions, including death, from mast cell degranulation.

The healing process was quite good. I am very pleased with STELFONTAⓇ (tigilanol tiglate injection)
10-year-old mutt Lila’s mast cell tumor could not be managed with surgery due to the location.
Lila's owner Olga Grove said, “I trust my veterinarian. She is very honest and straightforward so when she told me about STELFONTA I was willing to try it. We talked about the other medications that she would have to be on too."
"My experience was really good. If there was anything weird I would ask and my veterinarian would tell me if it was normal or not. It was almost like it was melting away or consuming itself, and even though we talked about everything I wasn’t quite prepared for it to fall off!”
Treatment with STELFONTA has been associated with cellulitis and severe tissue sloughing extending away from the treated site, resulting in extensive wounds that require additional treatment and prolonged recovery time.

We have been happy with STELFONTAⓇ (tigilanol tiglate injection)
Jamie Rauscher, RVT, is the 2022 president-elect of the National Association of Veterinary Technicians in America.
When one pet owner hesitated to have their dog undergo surgery to remove a mast cell tumor, STELFONTAⓇ was the logical choice. “Their third dog has a history of eating [foreign objects] and has had four surgeries. They (the pet owners) didn't want to put him through surgery anymore, and they were willing to [use STELFONTA]. So this worked out really well for them.”
In the end, both the healthcare team and the pet owners were satisfied with the results of STELFONTA. “We have been happy with it. Owners have done well with it.” Jamie would even choose STELFONTA to treat her dog. “I have a 13-year-old golden, and if she had a mass I think that I would do it, just to not put her through surgical recovery.”
Formation of wounds, possibly extensive, is an intended and likely response to treatment with STELFONTA, along with the associated swelling, bruising and pain; these wounds are expected to heal.

Turnip is doing AMAZING, thanks to STELFONTAⓇ (tigilanol tiglate injection), living her BEST life
Amy’s 8 year old French Bulldog, Turnip, was diagnosed with a mast cell tumor on her lip.
“The clinic told us about this treatment that was relatively new and we thought 'let’s give it a shot.' The first 24-28 hours were scary as her mouth swelled. The first week was a little worrisome watching it and then it was just fine.
Our veterinarian had us take pictures, document everything and send emails and they were awesome about following up and reassuring us.
I don’t know why more people don’t know about it [STELFONTA] and use it. It was just crazy. I would do it again without hesitation because it was so amazing, and it was so fast.”
Formation of wounds, possibly extensive, is an intended and likely response to treatment with STELFONTA, along with the associated swelling, bruising and pain; these wounds are expected to heal.

It's a great option for our patients with mast cell tumors
Kayla Brooks, a veterinary technician in Clarksville, TN said, “Initially when I heard about STELFONTAⓇ (tigilanol tiglate injection), I was skeptical, but also curious. I was very impressed with the end results. It's a great option for our patients with mast cell tumors. It's very important to communicate with the owner throughout the entire process, and ease their worries throughout the whole STELFONTA treatment process.
If it's red, bruised, anything like that, it can cause some alarms for some clients. So, we tell them to send us a picture, and we advise them appropriately.
As far as when the tumor disintegrates, I think it leaves a beautiful bed of granulation tissue, and then it heals wonderfully. We haven’t had any issues with healing. Usually within four to eight weeks, the dogs are pretty much back to normal, which is a much quicker recovery than even most surgeries.”
Formation of wounds, possibly extensive, is an intended and likely response to treatment with STELFONTA, along with the associated swelling, bruising and pain; these wounds are expected to heal.

Almost 20 patients treated with STELFONTAⓇ (tigilanol tiglate injection)
Dr. Jerrod Johnson said, “I have found STELFONTA to be a product that engages clients and they become excited watching it work before their eyes. It is one of the only products that mitigates some of the fear following a cancer diagnosis. I have and will continue to recommend STELFONTA for mast cell tumors that fall within the usage guidelines.
When discussing STELFONTA with clients, I stress the importance of the planning and the prep work. Administering the concomitant medications is exceedingly important to decrease risk of side effects caused by degranulation.
STELFONTA is a blessing for clients that are anesthesia averse.“
To decrease the risk of accidental self-injection, sedation of the dog may be necessary. Concomitant administration of a corticosteroid, an H1 receptor blocking agent and an H2 receptor blocking agent is required when treating with STELFONTA to decrease the potential for severe systemic adverse reactions, including death, from mast cell degranulation.

Amazing to watch
Sammi was concerned Ruby would have to be put down after her vet confirmed there wasn’t enough skin on Ruby’s leg, where the MCT was detected, to be cut out. But after a local treatment injected into the tumor, it was an easy decision to treat another MCT that appeared just months afterwards.
“It was amazing to watch because within a day it turned black and the tumor died. All we did was leave it, and it was amazing,” said Sammi. The second MCT took longer to heal, but Sammi noted “at first, it was a deep hole, but now it’s just a shiny scar about the size of a 20-cent piece. I really believe that this was a great option for Ruby. Within days, she was recovering and up again. She did not even need anesthetics.”

Back to being a typical Staffy bullet
Steve described how the wound where Shirley’s tumor used to be didn’t stop her from getting back to normal.
“Within a week of treatment, the lump became swollen and burst,” said Steve. “It smelt at first but within a week, she started coming good – even though the wound was there. It was amazing. Her recovery was incredible. She is now back to being a typical Staffy bullet – she’s nuts!”. Steve added that given the choice again, “we would definitely do this.”

Extended her life
“After the treatment, she was miserable for a few days, but within a week, she bounced back to normal, doing big walks and going running”, explained Katja. “Lou’s just had her eighth birthday. Without treatment, she wouldn’t have survived or enjoyed such an active life.”
“We intend to share many more years together,” said Katja, “so I don’t hesitate to continue with treatment when necessary.”

Even better than before
Nase’s owner Leanne spotted the lump on the back of his leg and went to the vet, who recommended an injectable treatment.
The treatment started to work quickly, “the lump swelled up within an hour and started to go dark. The vet explained this meant the treatment was working,” explained Leanne.
The best thing for Leanne was how Nase seemed to have even more energy than before. “Within a month, he was actually better after the treatment than beforehand. He wasn’t as tired and got his appetite back.”

Saved Daisy's leg
Daisy’s tumor was a lump that looked like a wart on her right leg.
Brigette explained she looked after the wound that formed after treatment. “Within a couple of hours, it started to go black and looked inflamed.”
“There was a bit of a smell, but not so bad that it bothered us,” explained Brigette. “It looked like a scab on top that started to fall away and left a clean crater.”
Today, you can hardly notice the tiny scar and Brigitte happily reported that “there’s no stopping Daisy now. The vet recommended this treatment because the alternative was limb amputation and I can’t imagine her quality of life, so this has been amazing. It’s prevented her from losing her leg.”

Thank you for recommending STELFONTAⓇ (tigilanol tiglate injection)
Juice's owner Heather Schultz said, "Juice is doing amazing and acting like a puppy again! I will never be able to thank Dr. Pearson enough for suggesting this treatment option. I was very concerned about Juice’s recovery time from surgery given his age (turned 12 in May!) and can’t believe how he recovered after STELFONTA. We were back to swimming and hiking 6 weeks after treatment."
"It's important for other pet owners to know that the first 10 days are hard, but it is SO WORTH IT."
Treatment with STELFONTA has been associated with cellulitis and severe tissue sloughing extending away from the treated site, resulting in extensive wounds that require additional treatment and prolonged recovery time.

Glad my veterinarian recommended an injectable
Piper was facing amputation to remove an MCT on her hind leg.
“That would have been devastating for Piper, who is such a high-energy dog,” explained Tammy. “Through treatment she was a bit more sleepy than usual, but within two weeks, she was back to her usual bouncy, bubbly self again.”
Tammy’s advice to fellow dog owners was simple. “I’d recommend it to anyone.”

Talk about the process with your veterinarian
Boston had a lump on his side that grew so quickly. Jacqui took him to the vet to investigate it.
Jacqui said it was reassuring to be able to talk to her vet as the tumor site went through the stages of healing. “It went gloopy at first, but the vet reassured us that was meant to happen.
Now a month and a half later, it’s just a small scar that you can hardly see and Boston’s like a new dog. He’d come up and say Hi, wag his tail and put his head in your lap. He gets excited and chases his ball and runs along the beach like his normal happy self.”

Bounced back to normal
Dennis recalled how quiet Mariah became after diagnosis, “she knew things weren’t right”. But after the treatment, it didn’t take long for Mariah to get her energy back. “She was down for the first week and didn’t want to do anything. But then she perked up and, within a month, she got more energy. Now she’s bouncing all over the place and keeps up with our other dog perfectly,” said Dennis.
He also commented on the healing of the tumor site, noting “gradually the skin grew back again and now it’s completely gone. Not even a mark, a dent – nothing. Completely gone.”

Treated what surgery could not
Barney’s tumor was on his bottom – a tricky place to treat surgically. Wendy explained why the veterinarian’s recommended treatment was an easy choice for them. “They could have tried surgery but may not have got all the tumor because it was so close to the bowel. Plus, as an older dog, there’s always the risks with anesthetics”, explained Wendy.
“The vet gave Barney the first injection, but a few weeks later, he had to have a second one to get the last few spots around the edge,” said Wendy.
Today, Barney is doing well, and Wendy is happy with the result. “You wouldn’t know he had cancer a month ago.”

11-year-old, well after treatment
Debra couldn’t be happier with the results of Sally’s treatment. “I’ve continued down this path because it’s not invasive,” explained Debra.
There’s always some initial discomfort, but as Debra explained, “they dry up really quickly within a couple of days and heal over.” Today, Sally is happy and as full of energy as an 11-year-old can be and Debra said Sally is still keen for walks and play. “If I pick up her lead, she’s all over me and ready to go.”

References
1. Data on file. Virbac Corporation.
STELFONTA® is the trademark of QBiotics Pty Ltd, used under license.
WARNING: SEVERE WOUND FORMATION IN HUMANS; EXTENSIVE WOUND FORMATION, MAST CELL DEGRANULATION, AND DEATH IN DOGS DUE TO MAST CELL DEGRANULATION
Human Safety
- Accidental self-injection of STELFONTA® may cause severe wound formation. To decrease the risk of accidental self-injection, sedation of the dog may be necessary (see Dosage and Administration, Human Warnings and Adverse Reactions).
Dog Safety
- Always administer a corticosteroid (e.g. prednisone or prednisolone), an H1 receptor blocking agent (e.g. diphenhydramine), and an H2 receptor blocking agent (e.g. famotidine) when treating with STELFONTA to decrease the potential for severe systemic adverse reactions, including death, from mast cell degranulation (see Contraindications and Dosage and Administration).
- Do not inject STELFONTA into subcutaneous mast cell tumors located above the elbow or hock (e.g. on the body, head, or neck). This may result in accumulation of necrotic debris in the subcutaneous space increasing the risk of systemic adverse reactions, including death, from mast cell degranulation (see Contraindications, Warnings and Adverse Events).
- Treatment with STELFONTA has been associated with cellulitis and severe tissue sloughing extending away from the treated site resulting in extensive wounds that require additional treatment and prolonged recovery times (see Warnings, Precautions and Adverse Events).
IMPORTANT SAFETY INFORMATION
Wear disposable gloves when cleaning the treated tumor site to avoid contact with any residual drug. Thoroughly wash your skin that comes in contact with the treated tumor site, wound, or wound discharge. Ensure your dog receives their prescribed medications to decrease the potential for severe, life-threatening adverse reactions. Amputation has been reported as a result of extensive swelling and wound formation. Monitor your dog during the healing process and contact your veterinarian if you notice excessive pain, lameness, tiredness, refusal to eat for more than one day, repeated vomiting or diarrhea, trouble breathing, changes to the treated tumor site (including increased or excessive swelling and bruising, extensive wound formation, increased irritation) or any other symptoms that concern you. For full prescribing information, contact Virbac at 1-800-338-3659 or click Product Insert to view.



