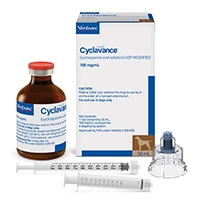
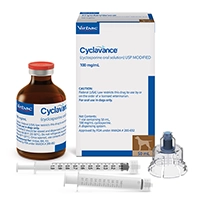
CYCLAVANCE™ (cyclosporine oral solution) USP MODIFIED
Give atopic dogs the cyclosporine they deserve with CYCLAVANCE™ oral solution.
Cyclavance is indicated for the control of atopic dermatitis in dogs weighing at least 4 lbs (1.8 kg) body weight.

Happiness is reliability
The effective cyclosporine you know and trust—IN LIQUID FORM
- Cyclosporine is proven to significantly relieve pruritus and reduce skin lesions in just 4 weeks (P < 0.001)1
- Cyclosporine maintains long-term beneficial effects with 1 dose every other day or twice weekly2
- Cyclosporine is recommended by the International Committee on Allergic Diseases of Animals (ICADA) for the treatment of atopic dermatitis in dogs3
- Blocks the release of inflammatory cytokines
- Exhibits strong anti-inflammatory and antipruritic effects
Mean cyclosporine plasma concentration (ng/mL) following administration at 5 mg/kg of CYCLAVANCETM (cyclosporine oral solution) USP MODIFIED and cyclosporine capsules4
.webp)
Happiness is precision in dosing
Eliminates the inefficiencies of dosing with capsules
- Liquid formulation allows for precise dosing at 5 mg/kg for all dogs
- Simple dosing makes it easy for the pet owner to administer the correct dose
- When compared with CYCLAVANCETM oral solution at 5 mg/kg, variance in dosing for cyclosporine capsules can range from -29% to +33%5,6
Cyclosporine capsules variance from 5 mg/kg ideal dose4,5+
.webp)
Happiness is confidence in delivery
Convenient and easy dosing to help promote compliance
- In 2 studies involving 320 individual tests comparing administration of CYCLAVANCETM oral solution with that of cyclosporine capsules (n=70):
- 99.3% of dogs easily accepted CYCLAVANCETM oral solution when administered directly in the mouth (vs 27.1% acceptance for cyclosporine capsules)7
- 98.3% of dogs consumed the entire dose of CYCLAVANCETM oral solution when mixed with a small amount of dry food (vs 2.2% consumption for cyclosporine capsules)7
- 2 vial presentations: 15 mL and 50 mL
- Syringe and adaptor cap for easy dosing with no leaks or spills
.webp)
Learn How to Correctly Apply the CYCLAVANCE Adaptor Cap
![23_icon_dark.webp]() Shop Virbac at Vetcove
Shop Virbac at Vetcove
You can shop all Virbac products on Vetcove and connect your distributor accounts to purchase and access rewards.
Pet Owner Information:
We have a separate site specifically tailored to pet owners with all the information you need to ensure your pets are taken care of to the highest degree! Click here to view this product's page on our pet owner website!
Important Safety Information
CYCLAVANCE™ (cyclosporine oral solution)(USP Modified oral solution): For use in dogs only. Wear gloves during and wash hands after administration. Gastrointestinal problems and gingival hyperplasia may occur at the initial recommended dose of CYCLAVANCE Oral Solution for Dogs. CYCLAVANCE Oral Solution for Dogs should be used with caution: 1) in cases with diabetes mellitus as it may cause elevated levels of serum glucose; 2) in dogs with renal insufficiency since the effect of cyclosporine use on dogs with compromised renal function has not been studied; 3) in simultaneous administration with drugs that suppress the P-450 enzyme system, such as azoles (e.g. ketoconazole), that may lead to increased plasma levels of cyclosporine. Killed vaccines are recommended for dogs receiving CYCLAVANCE Oral Solution for Dogs because the impact of cyclosporine on the immune response to modified live vaccines has not been evaluated. For full prescribing information, contact Virbac at 1-800-338-3659 or view the Product Insert.
References:
1. Steffan J, Parks C, Seewald W; North American Veterinary Dermatology Cyclosporine Study Group. Clinical trial evaluating the efficacy and safety of cyclosporine in dogs with atopic dermatitis. J Am Vet Med Assoc. 2005;226:1855–1863.
2. Steffan J, Favrot C, Mueller R. A systematic review and meta-analysis of the efficacy and safety of cyclosporin for the treatment of atopic dermatitis in dogs. Vet Dermatol. 2006;17:3–16.
3. Olivry T, DeBoer DJ, Favrot C, et al. Treatment of canine atopic dermatitis: 2015 updated guidelines from the International Committee on Allergic Diseases of Animals (ICADA). BMC Vet Res. 2015;11:210.
4. Data on file. Virbac Corporation.
5. CYCLAVANCE™ (cyclosporine oral solution) USP MODIFIED [product label]. Fort Worth, TX: Virbac AH, Inc.; 2020.
6. ATOPICA™ (cyclosporine capsules). Greenfield, IN: Elanco US Inc.; 2020.
7. Navarro C, Crastes N, Benizeau E, McGahie D. Voluntary acceptance and consumption of two oral ciclosporin formulations in dogs: two randomised, controlled studies. Ir Vet J. 2015;68:3.
Ingredients:
ACTIVE INGREDIENTS: Cyclosporine USP MODIFIED
All Rights Reserved. CYCLAVANCE is a trademark of Virbac S.A.